We see it every day. It powers our world. It gives us life. It’s the most familiar thing in the sky. And yet, we barely know it. It’s a giant, burning question mark hanging 93 million miles away. So, what is the sun made of?
You probably learned the simple answer in school: “mostly hydrogen and helium.” That’s true. It’s absolutely correct. But that simple answer glosses over a wild story—a story of incredible violence, ancient origins, and the exact same stuff that’s in your body right now. The sun’s composition isn’t just a static list. It’s the recipe for a 4.6-billion-year-old nuclear furnace that runs our entire solar system.
To really get our star, we have to look past that simple answer. We need to dig into the how and the why. How do we even know this? Where did all these ingredients come from? What happens to them under pressure we can’t even imagine?
Let’s peel back the layers.
More in Fundamental Concepts Category
Key Takeaways
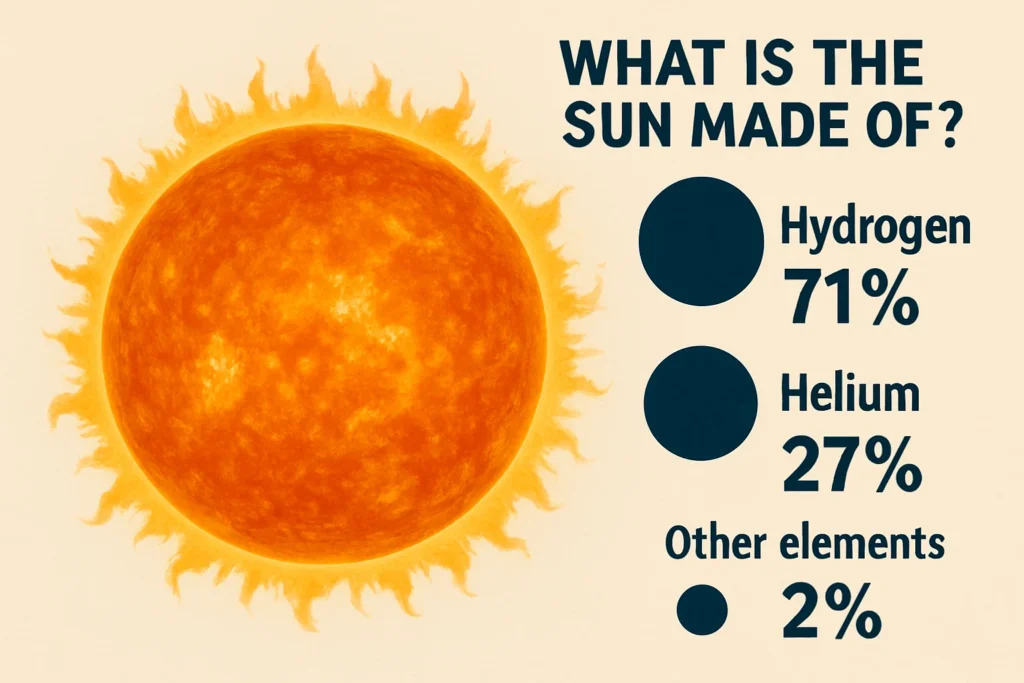
- It’s Mostly Two Things: The sun is almost entirely hydrogen (about 74% by mass) and helium (about 24%). That’s 98% of the star right there.
- The “Other Stuff” is Tiny: Everything else (what astronomers just call “metals”) is under 2%. This includes all the familiar stuff: oxygen, carbon, iron, you name it.
- We Read Its Light: We can’t visit the sun, so we analyze its light. A method called spectroscopy lets us read the unique “fingerprint” each element leaves in that light. It’s like a cosmic barcode.
- Composition is Fuel: The sun’s core is an engine. It fuses hydrogen into helium, and that process converts a tiny bit of mass into a whole lot of energy.
- We’re Made of Stardust: Our sun is a “second-generation” star. All its heavier elements (like carbon and iron) weren’t made in the sun. They were forged in giant stars that exploded billions of years ago.
So, What’s the Short Answer? (The Big Two)
Let’s get right to it. If you had a cosmic recipe for one star, our sun’s style, what’s in it?
It’s a surprisingly simple recipe.
By mass, the sun is about 74% hydrogen and 24% helium. Boom. That’s 98% of the entire star. Everything else… every bit of iron, every wisp of oxygen, every speck of carbon… is all crammed into the last 2%.
Hydrogen is element number one. The simplest, lightest, most common thing in the universe. Helium is number two. It’s the second simplest and second most common. It just makes sense that our sun, a pretty average star, is built from the universe’s two most common building blocks.
But that simple fact is also the most profound. That exact composition… that huge amount of hydrogen… is the reason the sun is a star. It’s not just what the sun is. It’s what the sun does.
The hydrogen is the fuel. The helium is the “ash.”
But How Can We Possibly Know This from 93 Million Miles Away?
This is the really clever part.
We can’t just send a probe to scoop up a piece of the sun. Of course not. The surface alone is 5,500 degrees Celsius (10,000°F). The core is 15 million degrees. Any ship we sent would vaporize instantly.
So, we do the next best thing.
We “read” its light.
The technique is called spectroscopy. It’s one of the most powerful tools in all of astronomy. It’s a way to find atomic fingerprints.
What is This ‘Spectroscopy’ You Speak Of?
You’ve seen basic spectroscopy in action. Shine white light through a prism, what happens? It splits into a rainbow. That rainbow is a “spectrum.”
When astronomers do this with sunlight, they find something fascinating. The sun’s rainbow isn’t perfect. It’s crossed by thousands of tiny, dark, vertical lines. These are “Fraunhofer lines,” named after the physicist who first studied them.
What are they? Gaps.
They’re specific colors, or frequencies, of light that are just… missing.
Are You Saying We Can Read the Sun’s “Barcode”?
Yes. That’s a perfect analogy.
Here’s how it works. Every element on the periodic table—hydrogen, helium, iron, anything—gets ‘excited’ when it’s heated. And when it’s excited, it absorbs or emits light at a very specific set of frequencies. Only at those frequencies.
It’s a unique, unchangeable atomic fingerprint.
The “glowing hot” part of the sun (the photosphere) creates a brilliant, continuous rainbow. But this light has to pass through the sun’s “cooler” upper atmosphere. The elements in that atmosphere—like hydrogen atoms—get excited and absorb their specific frequencies from the light.
By the time that light reaches Earth, it has those “gaps.”
Scientists look at that pattern of missing lines. They can match it, with perfect precision, to the known fingerprints of elements we have right here on Earth. That dark line right there? That’s hydrogen. That cluster of lines over here? Unmistakably iron.
This is how we know what is the sun made of. We read its barcode.
This technique is so powerful, it’s how we discovered helium. It was found on the sun in 1868. That’s 27 years before we ever identified it here on Earth. That’s why it’s named “helium,” after Helios, the Greek god of the sun.
What About the Other 2%? The Sun’s “Metals”
Okay, so 98% is hydrogen and helium. But what about that “other 2%”? This is where things get really interesting.
This 2% contains all the other 90-or-so natural elements. And in astronomy, this “other stuff” has a funny nickname.
Why Do Astronomers Call Everything “Metal”?
To an astronomer, the universe has three ingredients: hydrogen, helium, and “metals.”
It’s a bit of jargon, and it confuses a lot of people. When an astronomer says “metal,” they don’t mean the shiny stuff like iron or copper. To them, carbon is a metal. Oxygen is a metal. Even neon, the gas in a glowing sign, is a “metal.”
It’s just their shorthand for “everything else.” So a star’s “metallicity” is just a measure of how much “everything else” it’s got.
Okay, So What Are These “Metals” in the Sun?
So what’s in that 2% cocktail? The most abundant “metals” in the sun are, in order:
- Oxygen: By far the most common of the “metals,” making up almost 1% of the sun’s total mass.
- Carbon: The backbone of life on Earth. About 0.3% of the sun’s mass.
- Iron: A very important element. About 0.14% of the sun’s mass.
- Neon: Yes, the noble gas. About 0.12% of the sun’s mass.
- Nitrogen: Another crucial ingredient for life. About 0.1% of the sun’s mass.
- Silicon: The stuff that makes up sand and glass. About 0.07% of the sun’s mass.
After that, the amounts get truly tiny, but they’re there: magnesium, sulfur, and just about everything else on a periodic table.
That 2% might be a rounding error for the sun, but it’s everything to us. Without it, you get no rocky planets. No Earth. No life based on carbon and water. It’s all impossible.
If the Sun is Mostly Hydrogen and Helium, Where Did the Iron and Carbon Come From?
This is a great question. The answer connects us directly to the history of the entire universe.
The Big Bang kicked things off 13.8 billion years ago. It was hot, dense, and fast. In those first few moments, it only had time to make three elements. Hydrogen (a ton of it). Helium (a good amount). And a tiny, tiny trace of Lithium.
That’s it.
The early universe had no carbon. No oxygen. No iron.
So… where did the sun’s 2% of “metals” come from?
Are You Saying Our Sun is a “Second-Hand” Star?
Exactly. Our sun is a “second-generation” star. Maybe even third-generation.
The very first stars in the universe were born from those clean clouds of hydrogen and helium. They were massive. We’re talking hundreds of times the mass of our sun. And because they were so huge, they burned through their fuel at a crazy-fast rate.
Inside the fiery cores of those stars, nuclear fusion cooked up the first-ever batches of carbon, oxygen, nitrogen… all the elements up to iron.
And when those massive stars died, they did not go quietly. They exploded. We call them supernovas. These explosions were so powerful they forged even heavier elements (like gold and uranium) and blasted all of this new stuff out into space.
So the Sun Was Born from the Ashes of Other Stars?
Exactly. This cycle repeated for billions of years. Stars were born. They “cooked” hydrogen into heavier elements. They died, “seeding” the galaxy with this new, enriched material.
Then, about 4.6 billion years ago, one of these enriched clouds of gas and dust collapsed under its own gravity. This cloud had the original hydrogen and helium, but it also had all that new carbon, oxygen, and iron forged in long-dead stars.
Most of this cloud (99.8% of it) collapsed to form our sun. The leftover 0.2%, that “metal”-rich debris, formed everything else: the planets, the asteroids, the comets… and us.
That 2% isn’t just trivia. It’s our inheritance. The iron in your blood, the calcium in your bones, the carbon in your DNA… all of it was forged inside a star that died before our sun was even born.
We are, literally, stardust.
How Does the Sun’s Composition Create Its Energy?
Now we get to the engine room. The sun’s composition isn’t just a static fact; it’s the critical component of a machine.
The sun is not “on fire” like a log. A log fire is a chemical reaction. The sun’s power comes from a nuclear reaction. It remakes the very core of atoms.
This process is nuclear fusion. And it only happens because the sun is (a) massive and (b) made of hydrogen.
What’s Happening in the Sun’s Core?
Let’s journey to the center of the sun. The Core.
It’s almost impossible to wrap your head around. The pressure? 265 billion times Earth’s atmosphere. The temperature? 15 million degrees Celsius (27 million °F).
Under that crushing pressure and heat, normal atoms can’t exist. Electrons are stripped from their protons. This creates a superheated, churning soup of charged particles. We call it a plasma.
In this plasma, hydrogen nuclei (just single protons) move so fast and are packed so tightly that they overcome their natural repulsion. They slam into each other. They “fuse.”
Is This the Famous “E=mc²” Thing?
You bet it is. This is Albert Einstein’s most famous equation in action.
The main reaction in the core is the Proton-Proton Chain. Here’s the simple version:
- Four hydrogen protons (from the sun’s composition) are forced together.
- Through a few steps, they combine and transform.
- The end product is: one helium nucleus.
But here’s the miracle: If you weigh the four original hydrogen protons, and then weigh the one helium nucleus they create, the helium nucleus weighs just a tiny bit less (about 0.7% less).
That tiny bit of “missing” mass isn’t gone.
It’s been converted directly into a massive burst of pure energy. Mostly gamma rays.
This is happening on an unbelievable scale. Every second, the sun’s core fuses about 600 million tons of hydrogen into 596 million tons of helium.
That “missing” 4 million tons of mass is the energy. That’s the sunlight we feel on our faces. It’s the energy that powers photosynthesis, drives our weather, and makes life on Earth possible.
The sun is basically a star-sized hydrogen bomb, held together by its own gravity, in a perfect, continuous explosion.
Does the Sun’s Composition Change Depending on the Layer?
It sure does. The sun isn’t just one uniform ball of gas. It’s a complex, layered structure. Its composition, and what’s happening to it, changes dramatically as you move from the inside out.
The Core: The Ultimate “Hydrogen-to-Helium” Factory
We just visited the Core. It’s the only place in the sun hot and dense enough for fusion.
Because it’s been “burning” for 4.6 billion years, its composition is radically different from the rest of the star. While the sun overall is 74% hydrogen, the Core is now only about 34% hydrogen. The rest? Almost entirely 64% helium. That’s the ‘ash’ from 4.6 billion years of fusion.
The Radiative Zone: A Densely Packed “Photon Pinball Machine”
Outside the core is the Radiative Zone. It takes up a huge chunk of the sun’s interior. It’s still incredibly hot (2 to 7 million degrees) and unbelievably dense.
No fusion happens here. Its job is to transport the energy from the core.
Its composition is closer to the sun’s average: mostly hydrogen and helium. Energy from the core (as photons) tries to escape, but the plasma is so dense that a single photon can’t travel more than a few millimeters before it’s absorbed and re-emitted in a random direction.
It’s a “photon pinball machine.” A single photon from the core might take 100,000 years (or more!) just to bounce its way through this one layer.
The Convective Zone: A Boiling Pot of Plasma
This is the outermost interior layer, making up the top 30% or so of the sun.
Here, things have “cooled” (to a “mere” 2 million degrees) so the plasma acts more like a liquid. Think of a giant, boiling pot of water.
Hot blobs of plasma rise to the surface. They release their heat. They cool down, get denser, and sink. Then they go right back to the bottom to get reheated. It’s a constant, violent churn. This is what creates the “granules” (like bubbles) we see on the sun’s surface.
The Atmosphere (Photosphere, Chromosphere, Corona): What We “See”
This is the part we actually see. It’s what we analyze with spectroscopy.
- Photosphere: This is the visible “surface” of the sun. It’s the 5,500°C layer that emits most of the light we see. Sunspots appear here.
- Chromosphere: A reddish layer of gas just above the photosphere.
- Corona: The ghostly, super-hot (millions of degrees!) outer atmosphere. You can only see it during a total solar eclipse.
So when we measure the sun’s composition, we’re really measuring this atmosphere. Scientists work on a (very good) assumption: that this atmosphere has the same “recipe” as the original cloud our sun formed from.
Will the Sun’s Composition Change in the Future?
Absolutely. It’s changing right now.
Like we said, the sun is constantly turning hydrogen into helium. It’s a slow burn. The sun is massive, and it has enough hydrogen fuel in its core to last about 10 billion years.
It’s 4.6 billion years old, so it’s about halfway through its stable life.
But in about 5 billion years… things get dramatic.
What Happens When the Core Runs Out of Hydrogen?
In about 5 billion years, the sun’s core will have converted all its hydrogen into helium. Fusion will stop.
Gravity wins.
The dead helium core will collapse. As it collapses, it heats up. This new, intense heat will ignite the shell of hydrogen around the core.
This new “shell-burning” phase is unstable and ferocious. The new energy will push the sun’s outer layers outward.
The sun will swell. And swell. It becomes a Red Giant. It will grow so large it swallows Mercury. Then Venus. It might even get Earth.
Will the Sun Start Fusing Helium?
Yes. While the sun is swelling, that helium core keeps collapsing and heating. It gets hotter and hotter. When it hits a staggering 100 million degrees Celsius, a new fusion process ignites. It’s called the “Helium Flash.”
The sun will start fusing helium. Three helium atoms will slam together to become… one carbon atom.
For a while, the sun will be a “helium-burning” star, cooking helium into carbon and oxygen in its core.
And After That? The Sun’s Final Form
Our sun isn’t massive enough to fuse carbon. Once that helium is gone, the game is over.
The sun will become unstable. It sheds its outer layers into space. These layers—full of hydrogen, helium, and that new carbon—drift away. They form a beautiful, glowing “planetary nebula.”
Left behind, at the center, will be the sun’s dead, collapsed core. It will be an Earth-sized, super-dense ember made almost entirely of carbon and oxygen.
This is a White Dwarf. It’s no longer a star. No fusion is happening. It’s just a cooling cosmic ember, glowing with leftover heat, destined to fade to black over trillions of years.
So, What’s the Big Takeaway?
When we ask “what is the sun made of?” we’re really asking two questions.
The first answer is just a list: It’s hydrogen and helium, with a dash of “metals” like oxygen and carbon.
But the second, deeper answer is that the sun is a process. It’s a machine for turning hydrogen into helium. It’s a time capsule, holding recycled atoms from long-dead stars. And it’s our life-giving engine, turning a tiny bit of itself into all the energy that fuels our world.
The sun’s composition is more than just a fact. It’s our past, our present, and our future.
FAQ – What Is the Sun Made Of
What are the main elements that make up the Sun’s composition?
The Sun is primarily composed of hydrogen, making up about 74% of its mass, and helium, accounting for about 24%, with the remaining 2% consisting of various other elements known as ‘metals’ such as oxygen, carbon, and iron.
How do scientists determine what the Sun is made of from such a distance?
Scientists analyze the Sun’s light using a technique called spectroscopy, which reads the specific ‘fingerprints’ of elements in the light that has passed through the Sun’s atmosphere, creating a cosmic barcode that reveals its composition.
What do astronomers mean by calling other elements ‘metals’ in the Sun?
In astronomy, ‘metals’ refers to all elements other than hydrogen and helium, including oxygen, carbon, iron, and others, regardless of whether they are traditionally considered metals or gases on Earth.
Where do elements like carbon and iron found in the Sun come from?
These elements are the ‘metal’ remnants from previous generations of stars that were born, fused elements in their cores, and exploded as supernovae, seeding the universe with heavier elements which later became part of our Sun.
Will the Sun’s composition change in the future, and what happens when core hydrogen runs out?
Yes, the Sun’s composition will change over time. When the core hydrogen is exhausted, it will turn into a red giant and eventually shed its outer layers, leaving behind a dense core known as a white dwarf made mostly of carbon and oxygen.